Coulomb's law or Coulomb's inverse-square law is a law of physics describing the electrostatic interaction between electrically charged particles.
This law states that "The force of attraction or repulsion between two point charges is directly proportional to the product of magnitude of each charge and inversely proportional to the square of distance between them".
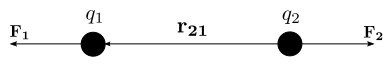
This law states that "The force of attraction or repulsion between two point charges is directly proportional to the product of magnitude of each charge and inversely proportional to the square of distance between them".

If the two charges have the same sign, the electrostatic force between them is repulsive;
if they have different sign, the force between them is attractive.
Mathematical equations:
Scalar form:
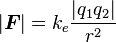 |
Vector form:
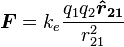
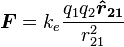
where k e = 1 ⁄ 4πε 0 (Coulomb's constant)
The Coulomb unit:
The Coulomb unit:
The coulomb is the SI derived unit of electric charge (symbol: Q or q).
It is defined as the charge transported by a steady current of one ampere in one second:
One coulomb is also the amount of excess charge on the positive side of a capacitance of one farad charged to a potential difference of one volt:
---------------------
Ref: Wikipedia

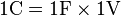
No comments:
Post a Comment